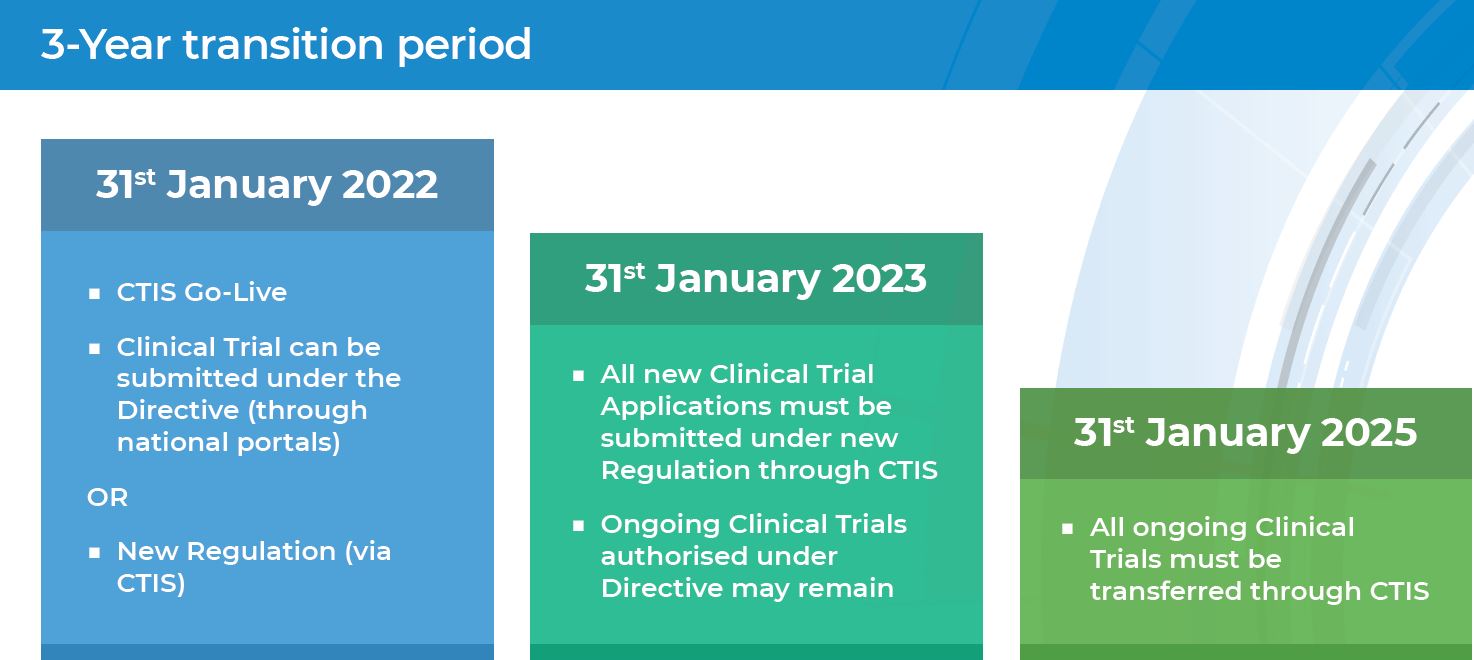
Starting from 31 January 2022 the EU Clinical Trials Regulation No 536/2014 (CTR) became applicable in all EU/ European Economic Area (EEA) Member States replacing the EU Clinical Trials Directive (2001/20/EC) (CTD). On the same day, the Clinical Trials Information System (CTIS), go-live version was opened for users to submit clinical trials on medicinal products for human use under coordination authorisation procedure.
Without a doubt, the CTR application has an impact on the clinical trials environment. Among others, the CTR harmonises the approval process for clinical trials, increases coordination between Member States, as well as maintains the highest level of standards for patient safety, and increases transparency of clinical trial information.
European Headquarters:
Via Giorgio De Sandre, 3
37135 Verona - Italy
Direct: +39 045 8222811
North American Headquarters:
8000 Regency Parkway, Suite 575,
Cary, NC 27518 – USA
Direct:+1 919 626 9882