Experience

CROMSOURCE has been ensuring clients in the biotechnology and pharmaceutical industries achieve their clinical research goals for over 25 years. As experts in the planning and delivery of clinical research, we understand the importance of time, cost and quality, and are able to guarantee these metrics are met. CROMSOURCE offers a comprehensive portfolio of services which can be tailored to meet the individual needs of each client.


The CROMSOURCE Medical Device team knows and understands medical device and diagnostics regulations; most of the staff has been through the regulatory process for clinical trial and product approval in the USA and EU many times. Therefore our operational team understands the regulatory implications of its actions and decisions, so your clinical study or regulatory submission is in safe, expert hands. We are also able to offer our clients the benefit of a large collective experience when formulating and implementing regulatory strategies and study designs, and CROMSOURCE’s mid-size and flat management structure means that this experience is not lost within a vast organisation.


After 25 years of experience in respiratory clinical research, CROMSOURCE is an internationally acclaimed expert in the area. That is why we were chosen to support the delivery of the UBIOPRED project in severe asthma conducted by academic and pharma partners in approximately 1000 children and adults. That is why our clients turn to CROMSOURCE again and again to support their work in respiratory medicine. They know that with CROMSOURCE they will receive a stable project team of professionals already deeply experienced in respiratory research. They know the CROMSOURCE team can provide expert insight into their development plan, and will go on to deliver their project according to the timelines and budget originally agreed.


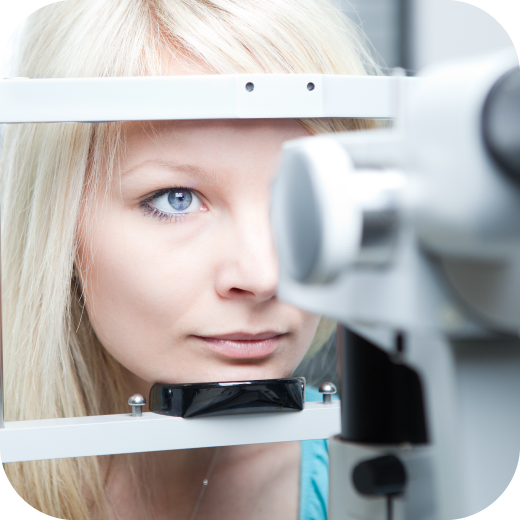
When you are developing products for the eye, you need specialist experience and expertise. You might also need a greater breadth of services and geographical coverage than small niche CROs can provide. CROMSOURCE combines the best aspects of a niche ophthalmology CRO with the stability and comprehensive in-house capabilities of a leading mid-size, full-service international CRO. Our geographic reach extends internationally, and our experienced team will expertly support your project in all applicable countries.
We have worked with CROMSOURCE for more than 10 years both in the EU and in the US. CROMSOURCE certainly lived up to their promises on our clinical trials. High quality. On time. On budget. We have already commissioned CROMSOURCE for our next project.